DNA guardians out of control
Researchers at the University of Bonn discover mechanism that leads to ANCA-associated vasculitis
Our own immune system can become the enemy when mechanisms that are actually protective get out of control. In ANCA-associated vasculitis, excessive inflammatory reactions lead to pulmonary hemorrhages that can be fatal if left untreated. Researchers at the University of Bonn, together with colleagues from Germany, the Netherlands, Switzerland and England, have deciphered a mechanism in mice and patients that leads to the severe disease. The results are now published in the Journal of Experimental Medicine.
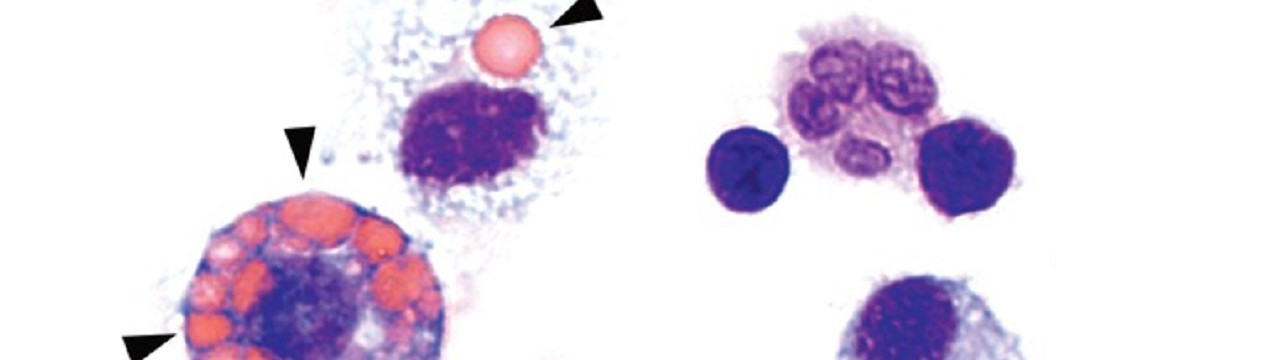
In ANCA-associated vasculitis, there is severe inflammation of the smaller and medium-sized blood vessels in the lungs. In addition, the skin and kidneys may also be affected. ANCA stands for “anti-neutrophil cytoplasmic antibodies,” which are antibodies produced by the body that target its own white blood cells. It is a rare, severe autoimmune disease that is often fatal if left untreated due to pulmonary hemorrhage.
Therapy involves the administration of drugs that suppress the immune system. Recently, attempts have also been made to block the inflammatory cascade with inhibitors. “The challenge in finding new therapies is that very little is known about the mechanisms that trigger the disease,” says Prof. Natalio Garbi of the Institute of Molecular Medicine and Experimental Immunology (IMMEI) at Bonn University Hospital.
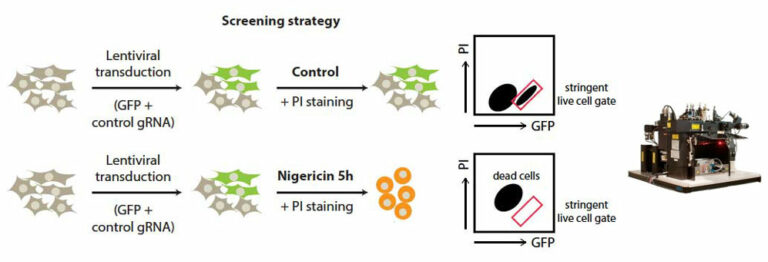
Together with colleagues from Germany, the Netherlands, Switzerland and the United Kingdom, the scientists have now discovered a mechanism responsible for the development of the disease in the form of the cGAS/STING/IFN-I signaling pathway. “We were able to show in experiments with mice that the symptoms of this autoimmune disease – such as pulmonary hemorrhage – improve when this signaling pathway is blocked with drugs,” says first author and doctoral student Nina Kessler from Natalio Garbi’s team. The study involved 31 patients with ANCA vasculitis and, as controls, 57 healthy individuals as well as a novel mouse model.
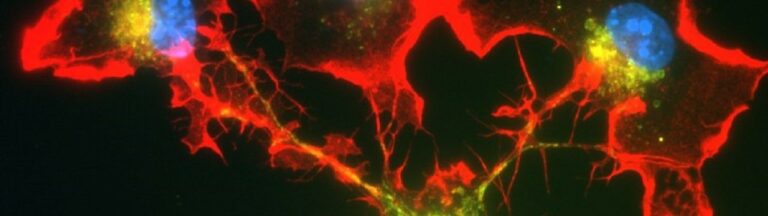
Normally, the genetic material DNA is located in the nucleus or mitochondria of cells. But if pathogens such as bacteria or viruses have taken up residence in the cell, they may leave behind a DNA trail in the cytosol that is detected by a special sensor called cGAS. This sentinel produces a molecule called cGAMP, which in turn activates the STING molecule. As a result, type 1 interferon (IFN-I) production occurs, leading to strong inflammation. This should prevent the pathogens from multiplying and even drive heavily infected cells into cellular suicide. […]
Participating Core Facilities: The authors acknowledge the support from the Flow Cytometry Core Facility.
Participating institutions and funding:
In addition to the Institute of Molecular Medicine and Experimental Immunology, the study involved the Institute for Clinical Chemistry and Clinical Pharmacology, the Medical Clinic III, and the Clinic for Radiotherapy and Radiation Oncology, at Bonn University Hospital, the Technical University of Dresden, the University of Cambridge, the University of Groningen, the University of Maastricht, Ludwig Maximilians University Munich, the University Hospital Aachen, the Helmholtz Center Munich, the Max Planck Institute for Neurobiology Martinsried and the Swiss Federal Institute of Technology in Lausanne.
The DFG TRR 237, funded by the German Research Foundation, and the Cluster of Excellence ImmunoSensation2 supported the project financially.
Publication: N. Kessler et al.: Monocyte-derived macrophages aggravate pulmonary vasculitis via cGAS/STING/IFN-mediated nucleic acid sensing; Journal of Experimental Medicine; DOI: 10.1084/jem.20220759