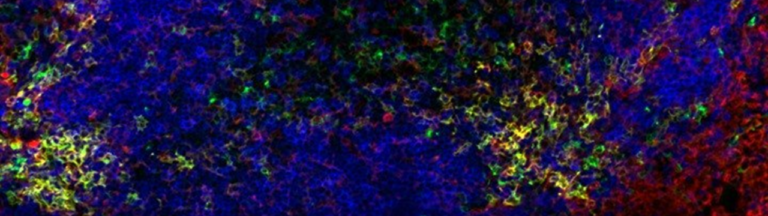
Stem cells organize themselves into embryoid
Study of mice by the University of Bonn could enable alternative methods to animal experiments in the medium term
Researchers at the University of Bonn have developed a method to generate embryo-like cell complexes from the stem cells of mice. The method provides new insights into embryonic development. In the medium term, it might also be suitable for developing tests for substances that could be harmful to fertility. The study is published in the prestigious journal Nature Communications.
It is still not fully understood how a mouse, a dog or a human being develops from a fertilized egg cell. The egg cell is capable of forming every type of tissue in the organism, whether it is bone, skin, muscle or the brain. Its daughter cells are genetically identical to it; so in principle they should be able to do the same. But in these cells, certain programs in the genetic material are activated very early on, which irreversibly determines their course of development.
This process must be coordinated down to the smallest detail. After all, this is the only way to ensure that the eyes form at the appropriate location on the face, while other cells very close by develop into the nasal cartilage. Surprisingly, however, there is no conductor wielding the baton. “Embryo development is largely based on self-organization,” explains Prof. Dr. Hubert Schorle of the Institute of Pathology at the University of Bonn. “Each cell releases messenger substances into its environment and thereby helps determine the fate of its neighbors.” It is as if in an orchestra everyone only pays attention to what the musicians around them are playing. And yet this would not result in a cacophony, but in Beethoven’s Ninth Symphony.
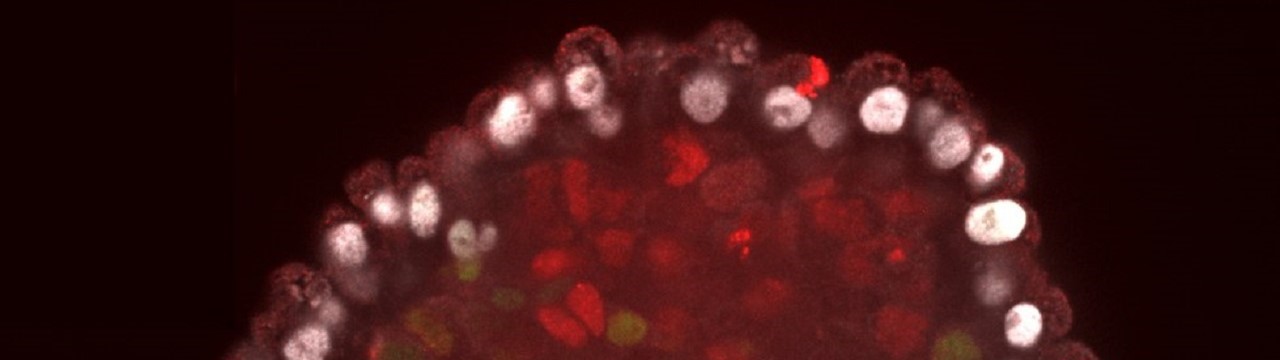
The current study allows new insights into these precisely coordinated processes. The researchers from Bonn succeeded in maturing embryonic stem cells (ES cells) from mice into a so-called embryoid. ES cells are pluripotent; different tissue types can form from them. Unlike omnipotent fertilized eggs, however, they are not all-rounders – so they no longer have every career open to them. “In addition to the actual embryo, the membrane that surrounds it and parts of the placenta also emerge from the egg,” Schorle explains. “ES cells, on the other hand, cannot form these tissue structures outside the embryo.” […]
Participating Core Facilities: The authors acknowledge the support from the Microscopy Core Facility. Furthermore, the Gene Editing Core Facility is affiliated with this study.
Participating institutions and funding:
The study was funded by the German Research Foundation (DFG).
Publication: J. Langkabel et al.: Induction of Rosette-to-Lumen stage embryoids using reprogramming paradigms in ESCs; Nature Communications, DOI: 10.1038/s41467-021-27586-w